The modern food supply chain is a marvel of industrial engineering, allowing for the global distribution of shelf-stable proteins that can remain viable for years. At the center of this system is canned tuna, a dietary staple valued for its convenience, nutritional density, and affordability. However, the very mechanism that ensures its longevity—the hermetic seal—is also the site of a rare but catastrophic vulnerability. When the integrity of this seal is compromised, whether through manufacturing defects or processing failures, it creates an environment conducive to one of the most lethal substances known to biological science: the botulinum neurotoxin. The recent wave of canned tuna recalls spanning 2025 and 2026 has brought this risk into sharp focus, revealing critical gaps in both container manufacturing and the logistics of food safety recalls.1
This report examines the multi-faceted nature of the botulism risk canned tuna recall, tracing the biological origins of the threat, the engineering failures behind recent incidents, and the complex regulatory framework designed to protect the public. By analyzing the 2025-2026 Genova and Tri-Union Seafoods recall events, we can better understand how a simple convenience feature like an “easy-open” lid can become a primary failure mode in an otherwise robust safety system.4
The Microbiology of Clostridium Botulinum: A Persistent Environmental Pathogen
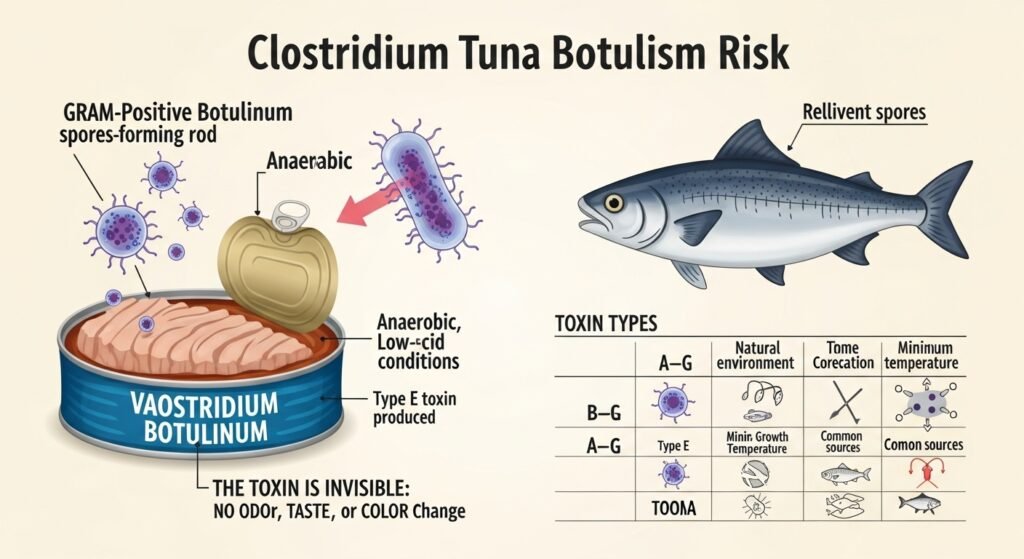
Understanding the risk associated with canned tuna recalls begins with the fundamental biology of Clostridium botulinum. This bacterium is an anaerobic, gram-positive, spore-forming rod that exists ubiquitously in the environment, particularly in soil and marine sediments.7 The organism is characterized by its ability to transition into a dormant spore state when environmental conditions are unfavorable. These spores are remarkably resilient, capable of surviving extreme temperatures, desiccation, and chemical exposure that would easily destroy most other pathogens.11
The danger posed by C. botulinum is unique in food safety. Unlike common pathogens like Salmonella or E. coli, which cause infection through bacterial colonization of the gut, C. botulinum causes intoxication through the ingestion of a pre-formed neurotoxin.7 This toxin is produced during the vegetative growth phase of the bacteria, which occurs only in the absence of oxygen (anaerobic conditions), a low-acid environment (pH > 4.6), and a favorable temperature range.8 Canned tuna, as a low-acid seafood product packaged in a vacuum-sealed container, provides the exact parameters required for toxin production if the sterilization process fails or the seal is breached.13
Toxin Serotypes and Marine Prevalence
There are seven distinct types of botulinum toxin, designated A through G. While all are potent, their impact on human health and their environmental distribution vary significantly.9 In the context of canned tuna recalls, Type E is of particular concern because it is the serotype most frequently associated with fish and marine environments.13
| Toxin Type | Environmental Reservoir | Minimum Growth Temp | Proteolytic Activity | Common Food Vehicles |
| Type A | Soil (Western US/China) | $10.0^\circ C$ ($50^\circ F$) | High (Produces Odor) | Vegetables, Fruits, Meat |
| Type B | Soil (Eastern US/Europe) | $3.3^\circ C$ ($38^\circ F$) | Variable | Meat, Some Vegetables |
| Type E | Marine/Freshwater Silt | $3.3^\circ C$ ($38^\circ F$) | Low (No Odor) | Fish, Seafood, Marine Mammals |
| Type F | Soil/Marine Silt | $3.3^\circ C$ ($38^\circ F$) | Variable | Rare (Varied) |
The distinction between proteolytic and non-proteolytic strains is a critical factor in consumer safety. Proteolytic strains (primarily Type A and some Type B) break down proteins during growth, often producing a foul odor, gas, or visible spoilage that may alert the consumer to danger.12 However, Type E—the strain most likely to be found in a botulism risk canned tuna recall—is non-proteolytic. It can grow and produce lethal levels of toxin without changing the food’s appearance, smell, or taste.9 This makes it an “invisible” killer, as the typical sensory checks for spoilage used by consumers are ineffective against it.18
The Spore Germination Cycle
The resilience of C. botulinum spores necessitates the high-temperature sterilization processes used in commercial canning. While vegetative cells are destroyed at temperatures near the boiling point of water ($100^\circ C$ or $212^\circ F$), the spores can survive these conditions indefinitely.11 To achieve “commercial sterility,” the industry employs a “12D reduction” target, which is a thermal process designed to reduce the population of the most heat-resistant spores by 12 log cycles.17 This typically requires internal temperatures of $116^\circ C$ ($240^\circ F$) to $121^\circ C$ ($250^\circ F$) maintained under pressure for a specific duration determined by the container’s size and the product’s density.12
The Mechanics of Contamination: Why Canned Tuna is Vulnerable
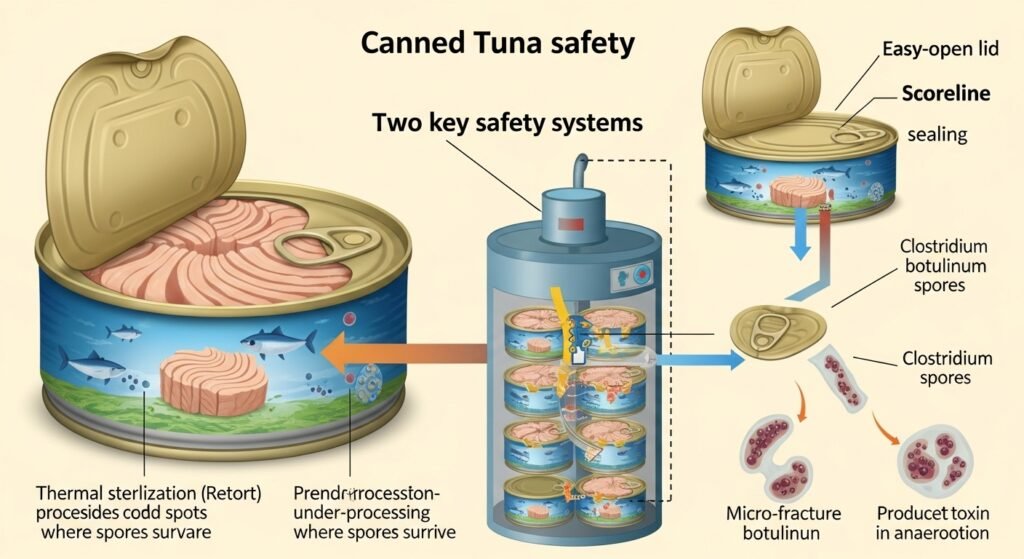
The safety of canned tuna relies on two independent but equally vital systems: the thermal sterilization (retorting) and the hermetic sealing of the container. A failure in either system can result in a catastrophic botulism risk.16
Retort Failure and Under-processing
In a commercial cannery, thousands of cans are processed simultaneously in large pressure vessels called retorts. The FDA’s mandates strict adherence to “scheduled processes” for low-acid foods.22 These processes are scientifically validated to ensure that even the most buried spore in the center of the can receives enough heat to be rendered non-viable.16
Under-processing occurs when the retort fails to reach the required temperature, fails to maintain it for the necessary time, or when “cold spots” occur within the vessel due to improper steam distribution.21 A notable historical example is the 2007 Castleberry’s Food Company recall, where a malfunctioning retort allowed C. botulinum spores to survive in millions of cans of chili sauce.21 In such cases, the bacteria are already inside the sealed, oxygen-free can; they simply wait for the product to cool to room temperature before they begin to germinate, multiply, and excrete toxin.7
Container Integrity and Post-Processing Contamination
Modern canned tuna recalls are increasingly linked to container integrity issues rather than sterilization failures. This is particularly true for the 2025-2026 events involving Genova and Tri-Union Seafoods.3 The transition from traditional cans to “easy-open” lids with pull-tabs has introduced a new mechanical failure mode.3
A hermetic seal is traditionally achieved through a “double seam,” where the lid (end) and the can body are interlocked in a two-stage rolling process that creates five layers of metal.26 The integrity of this seam is reinforced by a gasket compound that fills any microscopic gaps.28 However, an easy-open lid requires a “scoreline”—a thinned area of metal that allows the consumer to pull the lid off.26 If this scoreline is too deep, or if the metal becomes brittle during the manufacturing process, it can develop micro-fractures.26
When a can with a defective seal cools after retorting, a vacuum is created inside. This vacuum can “suck in” microscopic amounts of cooling water or environmental air.26 If C. botulinum spores are present in that environment, they enter the can. Once inside, the tiny breach may become clogged with product or oils, effectively re-sealing the can and restoring the anaerobic conditions required for the spores to thrive.3
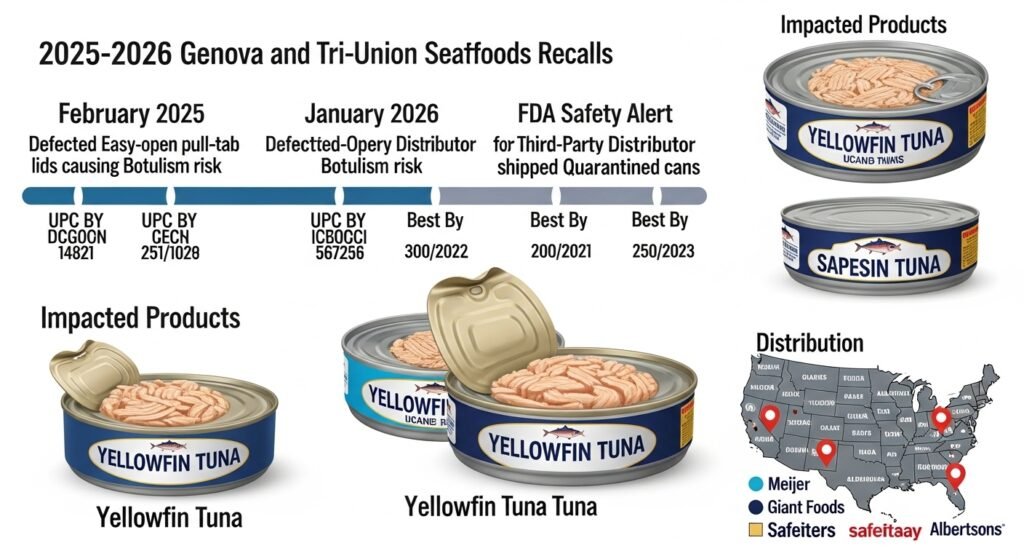
Case Study: The 2025-2026 Genova and Tri-Union Seafoods Recall
The most significant recent disruption in the canned tuna market illustrates a double failure: one of manufacturing and one of logistics. This event serves as the primary context for the current public concern regarding canned tuna recalls.1
Timeline of the Recall
The crisis began in February 2025, when Tri-Union Seafoods issued a voluntary Class I recall for several varieties of Genova and Van Camp’s canned tuna. The company had identified a manufacturing defect in the “easy-open” pull-tab lids supplied by a third party.3 The defect was described as a failure in the seal’s integrity over time, posing a direct botulism risk.3
However, the situation escalated in January 2026. The FDA released a safety alert revealing that a third-party distributor had inadvertently shipped quarantined cases of the recalled product to retailers across nine states.2 This logistical error meant that products known to be potentially lethal were once again available on store shelves nearly a year after they were first identified as hazardous.1
Impacted Products and Distribution
The 2026 redistribution affected specific lots of yellowfin tuna, often sold in multi-packs, which are popular for high-volume consumers.
| Brand | Product | UPC | Can Code | Best By Date |
| Genova | Yellowfin Tuna in Olive Oil (5 oz 4-Pack) | 4800073265 | S84N D2L | 01/21/2028 |
| Genova | Yellowfin Tuna in Olive Oil (5 oz 4-Pack) | 4800073265 | S84N D3L | 01/24/2028 |
| Genova | Yellowfin Tuna in EVOO w/ Sea Salt (5 oz) | 4800013275 | S88N D1M | 01/17/2028 |
The redistribution was concentrated in major retail chains, including Meijer (across the Midwest), Giant Foods (in the Mid-Atlantic), and several chains in California, such as Safeway and Albertsons.1 The fact that these cans were “Best if Used By” 2028 underscores the long-term risk; a consumer could have purchased a defective can in 2026 and not opened it until years later, by which time the toxin levels could be maximal.3
Pathophysiology of Botulism: Why It Is a Medical Emergency
Botulism is not a typical case of “food poisoning.” It is a neuroparalytic syndrome that requires intensive medical intervention for survival. The botulinum toxin is a zinc-dependent endopeptidase that targets the machinery of neurotransmission.35
Mechanism of Nerve Blockade
The toxin specifically targets the cholinergic nerve endings. Upon entering the body, it binds to the presynaptic membranes of the neuromuscular junction.35 Through a process of receptor-mediated endocytosis, the toxin enters the nerve cell. Once inside, it cleaves the SNARE (Soluble NSF Attachment Protein Receptor) proteins.35 These proteins are responsible for docking the vesicles containing acetylcholine to the cell membrane. Without SNARE proteins, acetylcholine cannot be released into the synaptic cleft, and the muscle receives no signal to contract.35
The result is flaccid paralysis. Because the toxin binds irreversibly, the nerve ending is essentially “dead.” Recovery only occurs when the body grows new nerve terminals, a process that takes weeks or even months.10
Clinical Presentation and Symptoms
The hallmark of foodborne botulism is a symmetric, descending paralysis. It always begins with the cranial nerves and moves downward.10
| Phase | Symptoms | Medical Significance |
| Early (12–36 hours) | Blurred vision, double vision (diplopia), drooping eyelids (ptosis).2 | Often misdiagnosed as a stroke or Bell’s Palsy.21 |
| Intermediate | Slurred speech, difficulty swallowing, dry mouth.14 | Indicates progression to more critical cranial nerves.10 |
| Advanced | Muscle weakness in neck/arms, followed by respiratory distress.9 | The most common cause of death is respiratory failure.8 |
| Gastrointestinal | Nausea, vomiting, abdominal pain (sometimes present).2 | Can occur before or alongside neurological signs.14 |
Crucially, botulism does not cause a fever or loss of consciousness.9 Patients remain fully aware as their body’s muscles slowly cease to function, making it a particularly harrowing clinical experience.36
Clinical Management and the Strategic National Stockpile
Because of its rarity and severity, botulism is a reportable public health emergency. When a physician suspects botulism, they must immediately contact state or local health departments, which in turn coordinate with the to obtain antitoxin.35
Heptavalent Botulinum Antitoxin (HBAT)
The primary treatment for non-infant botulism is Heptavalent Botulinum Antitoxin (HBAT). Derived from equine (horse) serum, HBAT contains antibody fragments that bind to and neutralize all seven known serotypes of the toxin circulating in the blood.35
However, HBAT is only effective against toxin that has not yet bound to nerve endings.35 It can stop the progression of the disease but cannot reverse paralysis that has already occurred. This is why “early administration” is the most significant factor in patient outcomes.36 Patients treated within two days of symptom onset typically spend 10 fewer days in the hospital than those treated later.40
Supportive Care and Long-Term Recovery
For patients whose respiratory muscles are already paralyzed, the only treatment is mechanical ventilation. In severe cases, patients may require a ventilator for months while their nerves regenerate.10 This long-term intensive care carries its own risks, including secondary infections, blood clots, and muscle atrophy.36 Even after being weaned off a ventilator, survivors often face months of physical and occupational therapy to regain basic motor functions.14
Regulatory Oversight: Classifying and Managing Recalls
The FDA and USDA employ a standardized classification system to communicate the urgency of food recalls to the public and the industry.41
The Recall Hierarchy
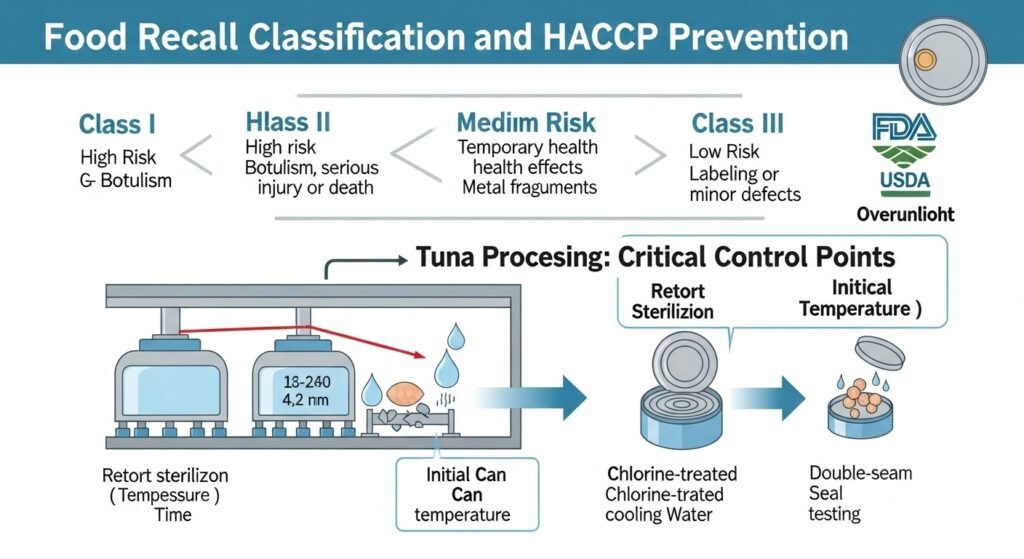
- Class I Recall: Reserved for “high-risk” situations where there is a reasonable probability that eating the food will cause serious health problems or death.41 Any botulism risk canned tuna recall is automatically Class I.41
- Class II Recall: Used for “medium-risk” situations where the product might cause temporary or medically reversible health problems, such as a minor undeclared allergen or the presence of small metal shavings.41
- Class III Recall: Applied to “low-risk” situations where the product violates labeling or manufacturing laws but is unlikely to cause adverse health consequences, such as an incorrect weight label or a minor container defect that does not compromise the seal.41
The Role of HACCP in Prevention
Hazard Analysis Critical Control Point (HACCP) is the preventative framework used by seafood processors. Under(https://www.fda.gov/food/hazard-analysis-critical-control-point-haccp/seafood-haccp), manufacturers must identify every point in the process where a hazard could be introduced and implement “Critical Control Points” (CCPs) to manage them.12
For a tuna processor, the retorting phase is the primary CCP for C. botulinum. This involves monitoring not just the temperature of the retort, but also the “initial temperature” of the can before it enters, the pressure, and the consistency of the heat penetration.12 Post-processing CCPs include the monitoring of the cooling water’s chlorine levels (to ensure it is sterile) and the periodic destructive testing of can seams to ensure they meet the 1.2x thickness requirement for a safe seal.26
Historical Trends: Why Modern Canning is Still Safe
Despite the recent sensational nature of canned tuna recalls, it is important to place these events in a broader historical context. The safety record of the commercial canning industry remains one of the greatest successes of public health.17
The Shift from Home to Industrial Safety
In the early 20th century, botulism was a relatively common cause of death, primarily linked to home canning where families used “boiling water baths” instead of pressure canners for low-acid vegetables.7 Because water boils at $212^\circ F$ ($100^\circ C$), it is physically impossible to kill C. botulinum spores without the added pressure of a retort.11
Today, home canning still accounts for approximately 29% of foodborne botulism cases, while commercially canned foods account for only about 10%.21 The rarity of these events is what makes the 2025-2026 Tri-Union recall so significant; it represents a failure of a system that normally operates with near-perfect reliability.21
Lessons from the 1963 Detroit Outbreak
The modern regulatory landscape for tuna was largely forged in the aftermath of the 1963 Detroit outbreak. Three women became ill and two died after a luncheon involving commercially canned tuna salad.48 The investigation revealed that Type E spores had survived in a specific batch of cans processed in a California cannery. This event led directly to the implementation of the strict FDA thermal processing records and the requirement for canners to be registered with the federal government.21
Identifying Recalled Products: A Guide for Consumers
In the event of a botulism risk canned tuna recall, consumers cannot rely on their senses to detect the toxin. Instead, they must rely on the data printed on the container.1
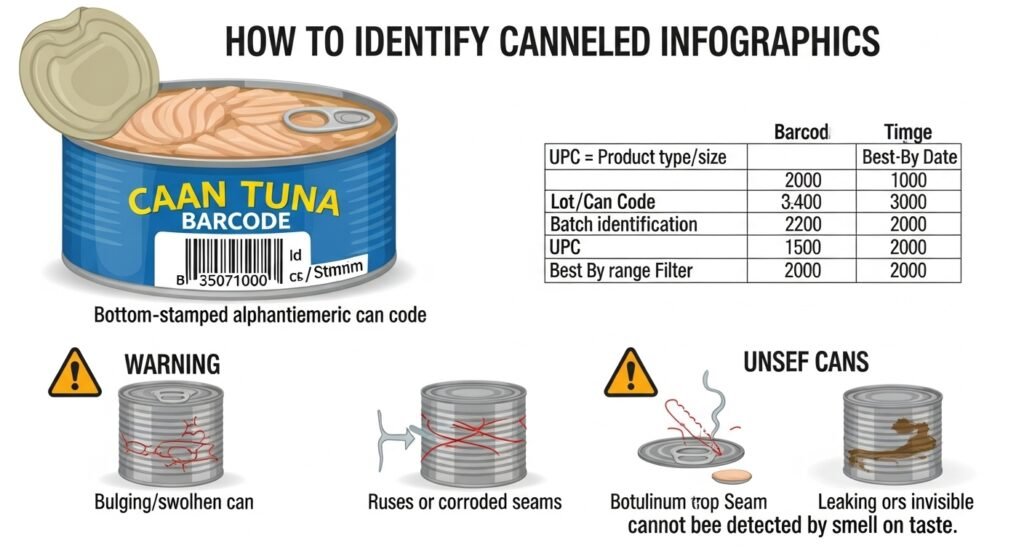
How to Read Can Codes
Every can of tuna has a alphanumeric code stamped on the bottom. This code allows the manufacturer to trace the product back to a specific day, shift, and even the specific retort vessel used.16
| Code Element | Meaning | Relevance to Recall |
| UPC (Barcode) | Product Type/Size | Identifies if the brand and size match the alert.5 |
| Lot/Can Code | Batch Identification | The most precise way to know if your specific can is dangerous.5 |
| Best By Date | Quality Window | Used as a secondary filter to narrow down the affected time period.3 |
Visual Indicators of Failure
While Type E toxin is “invisible,” the mechanical failures that allow it to develop often leave clues. Consumers should discard any can that exhibits:
- Bulging or Swelling: This indicates that gas-producing bacteria have contaminated the can. Even if it is not C. botulinum, the seal is clearly compromised.7
- Rust or Corroded Seams: Rust can eat through the metal, creating micro-pores.31
- Dented Seams: A dent on the top or side seam (where the lid meets the body) is far more dangerous than a dent on the side of the can, as it can “spring” the seal.28
- Leakage or Sticky Residue: This is a definitive sign of a seal failure.3
Future Outlook: Technology and Logistical Reform
The 2026 redistribution error has sparked a debate among food safety experts regarding the need for “smart” recall systems. The current system relies on manual quarantine and the hope that third-party warehouses follow instructions.
Technological Advancements in Inspection
Manufacturers are now moving toward 100% automated inspection. Technologies such as ultrasonic pulse-echo systems can scan the scorelines of easy-open lids at speeds of over 1,000 cans per minute, detecting metal fractures that are invisible to the human eye.30 Similarly, “Integra” systems use sensors on the seaming machine to monitor the pressure and height of every single double seam as it is formed, automatically rejecting any can that deviates from the “perfect” safety profile.29
Digital Traceability
The future of recall management likely lies in blockchain or centralized digital ledgers. If every case of tuna was tracked with a unique digital identifier (UID), a retailer’s point-of-sale system could automatically block the sale of a recalled item, even if a distributor accidentally put it back on the truck.44 This would create a digital “safety net” that prevents human error from endangering the public.43
Conclusion: Ensuring Safety Without Panic
The risks associated with canned tuna recalls and Clostridium botulinum are real and severe, but they are also manageable. The 2025-2026 Genova and Tri-Union Seafoods events have highlighted that our safety systems are only as strong as their weakest link—whether that is a pull-tab lid or a warehouse worker’s mistake. However, the fact that these issues were identified, publicized, and managed demonstrates the resilience of our public health infrastructure.
For the consumer, the message is one of empowered vigilance rather than fear. Canned tuna remains one of the safest and most efficient sources of protein available, provided one respects the basic rules of container integrity. By checking lot codes against official FDA alerts and following the “when in doubt, throw it out” rule for damaged cans, individuals can enjoy the benefits of this pantry staple without undue risk. Food safety is a shared responsibility, and an informed public is the final, most important line of defense against the silent threat of botulism.7
Frequently Asked Questions
No. In fact, most recalls are categorized as “Class II” or “Class III” by the FDA, which usually involve non-life-threatening issues like undeclared allergens (e.g., soy or milk), incorrect labeling, or minor quality defects. Botulism-related recalls are typically “Class I” (the most serious) and are rare, preventative measures taken when manufacturing records show a potential failure in the sterilization process.
While the botulinum toxin is heat-labile (meaning it can be neutralized by boiling for 10 minutes at temperatures above 85°C/185°F), health authorities strongly advise against it. If a can is part of a recall or shows signs of spoilage (like bulging or leaking), do not open it, taste it, or attempt to cook it. Even a microscopic amount of toxin absorbed through the skin or accidentally inhaled can be dangerous.
A “Best By” date indicates quality over time, whereas a Lot Code (or Batch Code) is a specific string of numbers and letters used by the factory to identify the exact minute, day, and machine used to produce that can. Lot codes are usually stamped in ink directly onto the metal of the lid or the bottom of the tin. During a recall, only specific lot codes are affected, not every can on the shelf.
When Clostridium botulinum bacteria grow inside an airtight can, they often produce gas as a metabolic byproduct. This gas creates internal pressure, causing the flat ends of the can to swell or “bulge.” A bulging can is a primary visual red flag for bacterial activity and should never be opened.
It depends on the location and severity of the dent:
Safe: Small, shallow dents on the side of the can that do not have sharp creases.
Unsafe: Any dent located on the top or bottom seam (the rim), or dents so deep that they have a sharp, “V” shaped crease. These can create microscopic fractures in the metal or seal, allowing oxygen and bacteria to enter.
If you have consumed a product that was later recalled for botulism risk, stay calm but remain vigilant. Symptoms usually appear within 12 to 36 hours. Monitor yourself for double vision, slurred speech, or difficulty swallowing. If any neurological symptoms appear, go to the emergency room immediately and inform them that you may have been exposed to botulinum toxin.
